Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
Research — December 18, 2025
By Digvijay Todkar
Mirum Pharmaceuticals Inc. (NASDAQ: MIRM) recently announced that it has entered into a definitive agreement to acquire privately held Bluejay Therapeutics in a deal worth up to about $820 million, marking a strategic push into viral liver disease and expanding its growth options beyond rare pediatric cholestatic disorders.
The deal centers on brelovitug, an experimental antibody designed to block the surface antigen shared by hepatitis D and hepatitis B. Hepatitis D is a severe form of viral hepatitis with no widely approved targeted therapies, leaving a meaningful gap in treatment despite a relatively small patient population. For Mirum, the drug offers a potential new revenue stream in a high-unmet-need niche that could support premium pricing if approved.
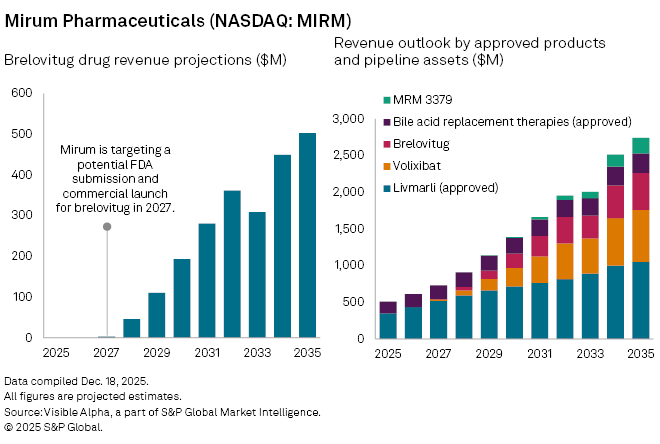
Analysts assign a 65% probability of success to brelovitug. Visible Alpha consensus forecasts shows risk-adjusted sales of $4 million in 2027, pending regulatory approval, rising to $46 million in 2028 and $110 million in 2029. Longer term, sales are projected to reach $503 million by 2035, when the drug could account for roughly 19% of Mirum’s total revenue.
The acquisition complements Mirum’s existing commercial portfolio, which includes Livmarli, its flagship therapy for rare cholestatic liver diseases, as well as bile acid replacement therapies Chenodal and CHOLBAM.
Mirum is targeting a potential FDA submission and commercial launch for brelovitug in 2027.
Beyond brelovitug, Mirum’s pipeline includes volixibat, an ileal bile acid transporter inhibitor currently in phase 2b trials for two forms of cholangitis, and MRM-3379, a phase 2 PDE4D inhibitor for fragile X syndrome.
This article was published by Visible Alpha, part of S&P Global Market Intelligence and not by S&P Global Ratings, which is a separately managed division of S&P Global.
Content Type
Products & Offerings
Segment