Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Professional Services
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Professional Services
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
Research — OCTOBER 02, 2025
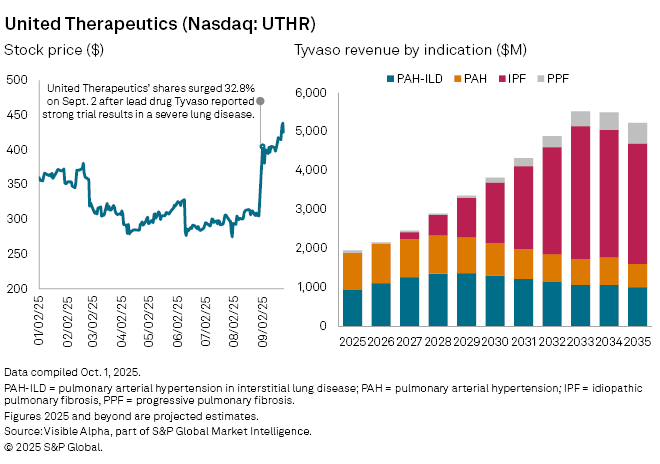
United Therapeutics Corp. (NASDAQ: UTHR) shares climbed in early September following positive regulatory designations and encouraging phase 3 data for Tyvaso in early September, its inhaled treprostinil therapy, in idiopathic pulmonary fibrosis (IPF) — a progressive lung disease with limited treatment options. If approved, Tyvaso would become the first inhaled therapy for IPF, strengthening its market advantage.
Tyvaso is already a cornerstone of United’s portfolio, approved for pulmonary arterial hypertension (PAH), interstitial lung disease (ILD), and progressive pulmonary fibrosis (PPF). Analysts expect the IPF indication to become the company’s most important growth driver, with Visible Alpha consensus showing analysts assign a 76.4% probability of success.
The company is advancing its pivotal Teton-1 study, with final results expected in early 2026. Management also intends to meet the US Food and Drug Administration by late 2025 to explore expedited review, potentially accelerating commercial launch.
Consensus forecasts suggest Tyvaso in IPF could deliver $172 million in risk-adjusted sales in 2027, the first expected full year on the market, before scaling to $527 million in 2028. By 2030, IPF-related revenue is projected at $1.5 billion, rising to $3.4 billion by 2033 — roughly 42% of Tyvaso’s sales and 32% of United’s total revenue. Across all indications, Tyvaso is expected to generate $1.9 billion in 2025, accounting for 60% of United’s group revenue.
United’s overall revenue is forecast to grow steadily, with analysts projecting a 12% year-on-year increase to $3.2 billion in 2025.
This article was published by Visible Alpha, part of S&P Global Market Intelligence and not by S&P Global Ratings, which is a separately managed division of S&P Global.
Content Type
Products & Offerings
Segment
