Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Professional Services
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Professional Services
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
Research — SEPTEMBER 23, 2025
By Urvi Shah
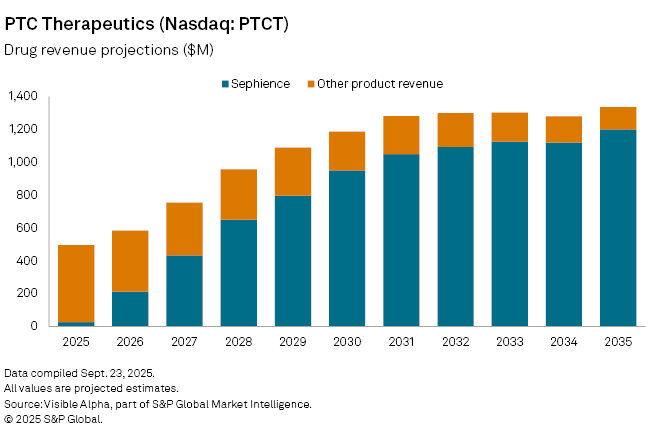
PTC Therapeutics Inc. (NASDAQ: PTCT) is pinning its next phase of growth on Sephience, a newly approved treatment for phenylketonuria (PKU), a rare inherited metabolic disorder. The drug, cleared by regulators in the US and EU this summer, was launched in Germany in July, with a US rollout expected in the coming months. Based on Visible Alpha consensus, analysts forecast sales of about $27 million in 2025, rising to $212 million the following year, with blockbuster revenues of more than $1 billion projected by 2031. At peak, Sephience could generate $1.2 billion in global sales and account for more than 60% of the pharma company’s revenue, giving PTC its first blockbuster franchise.
The launch marks a significant shift for PTC, which has until now relied on a narrow portfolio of rare disease drugs. Sephience offers once-daily dosing and improved convenience over BioMarin Pharmaceutical’s (NASDAQ: BMRN), Kuvan and Palynziq, the dominant therapies for PKU. Success would strengthen PTC’s foothold in a market where competition has historically been limited, while also reducing its reliance on products approaching the end of their patent life such as like Translarna and Emflaza.
The company, however, faces lingering questions over its broader pipeline. The US Food and Drug Administration recently issued a Complete Response Letter for vatiquinone, its Friedreich’s ataxia candidate, citing the need for additional data. The setback underscores the risks in PTC’s development portfolio and places greater pressure on Sephience to deliver.
This article was published by Visible Alpha, part of S&P Global Market Intelligence and not by S&P Global Ratings, which is a separately managed division of S&P Global.
Content Type
Products & Offerings
Segment