Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Professional Services
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Professional Services
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
Research — SEPTEMBER 24, 2025
By Jueeli Kadam
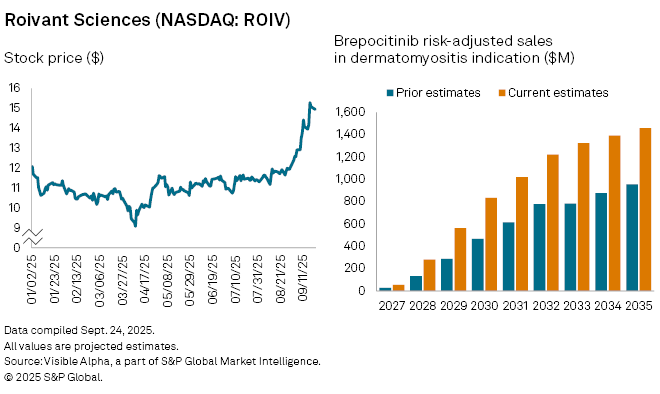
Shares of Roivant Sciences Ltd. (NASDAQ: ROIV) rallied after the company reported positive late-stage trial results for brepocitinib, an oral treatment for dermatomyositis, a rare autoimmune disorder that causes severe muscle weakness and skin inflammation. The drug is being developed through Priovant Therapeutics, a subsidiary of Roivant.
Priovant plans to file for US regulatory approval in the first half of 2026, setting up brepocitinib as a potential first-in-class oral therapy in a market where treatment options are limited. Currently, only one approved therapy exists for the condition, and no other late-stage oral candidates are in development.
Visible Alpha consensus shows analysts have sharply raised their forecasts following the positive result announcement. Consensus estimates for brepocitinib’s dermatomyositis indication now stand at $54 million in risk-adjusted sales in 2027, nearly doubling from expected $28 million prior to the announcement. Analysts also lifted the probability of approval from 67% to 86%, reflecting greater confidence in the drug’s regulatory prospects. Analysts project the drug could reach blockbuster status, with $1 billion in sales by 2031 in dermatomyositis indication and peak global revenues of $2.7 billion by 2039.
Beyond dermatomyositis, brepocitinib is being studied for other rare and orphan autoimmune conditions, raising hopes it could become a cornerstone therapy.
This article was published by Visible Alpha, part of S&P Global Market Intelligence and not by S&P Global Ratings, which is a separately managed division of S&P Global.
Content Type
Products & Offerings
Segment