Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
S&P Global Offerings
Featured Topics
Featured Products
Events
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Professional Services
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
Financial and Market intelligence
Fundamental & Alternative Datasets
Government & Defense
Professional Services
Banking & Capital Markets
Economy & Finance
Energy Transition & Sustainability
Technology & Innovation
Podcasts & Newsletters
26 Aug, 2025
Shares in Insmed Inc. (NASDAQ: INSM) have rallied nearly 20% since the US Food and Drug Administration approved Brinsupri (brensocatib), the company’s treatment for non-cystic fibrosis bronchiectasis (NCFB). The drug is approved for adults and children aged 12 and older, offering the first therapy specifically authorized for the condition, which until now has relied on airway clearance, antibiotics, and exercise.
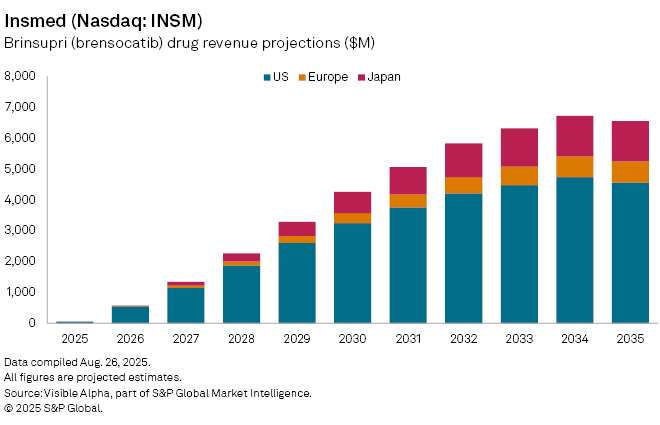
Analysts view Brinsupri as a potential standard of care for NCFB, given it is the first-in-class DPP-1 inhibitor in the respiratory field. Visible Alpha consensus forecasts suggest first-year sales could reach $52 million, climbing to $568 million by 2026, with peak global revenues projected at $6.3 billion by 2034.
Insmed has also filed for regulatory approval with the European Medicines Agency (EMA) and the UK’s MHRA, with commercial launches expected in 2026. In the US, Brinsupri is already available via a specialized pharmacy network. For investors, the approval not only validates Insmed’s pipeline strategy but also opens a sizeable, previously untapped market, positioning the company for strong revenue growth over the next decade.
Insmed’s total revenue is projected to rise 31% year-on-year in 2025 to $478 million. Analysts anticipate revenue could more than double in 2026 to $1 billion, driven largely by Brinsupri.
– Learn about Visible Alpha | S&P Global.
– Explore Visible Alpha Add-On for CapIQ Pro.
– Learn about Visible Alpha BioPharma.
– Access Visible Alpha estimates on Insmed.